Appendix A
Calculations
A.1 Calculation of the Water Vapor Pressure
The water vapor pressure of seawater is generally calculated from seawater temperature and salinity. We used the method given by Weiss and Price (1980) in which the authors provide an equation to assess the saturation water vapor pressure of seawater over the temperature range 273 to 313 K and the salinity range 0 to 40:
![]() ,
,
where psw is the water vapor pressure (in atm), T is the temperature (in K), and S is the salinity on the Practical Salinity Scale.
A.2 Calculation of fCO2 for Moist Air Conditions
The first step in the calculation procedure for final fCO2 values, either for the atmosphere or for surface seawater, is the calculation of the fCO2 for moist air from the measured mole fraction (xCO2) in dry air. Weiss and Price (1980) give the theoretical basis for this calculation based on equations given by Guggenheim (1967) for calculating fugacities in binary mixtures:
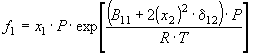 ,
,
where x1 is the mole fraction of pure gas 1, x2 is the mole fraction of pure gas 2, P is the total pressure, R is the gas constant, T is the absolute temperature, and d 12 is defined by
![]() ,
,
where B11 is the virial coefficient for interaction between pure gas 1 molecules, B22 is the virial coefficient for interaction between pure gas 2 molecules, and B12 is the virial coefficient for interaction between molecules of gas 1 and 2. For calculating fCO2, gas 1 is here considered as the analyte gas (CO2) and gas 2 as dry air. The mole fraction x2 of dry air in this mixture is approximately equal to 1 for the analyte concentrations considered here. To calculate the fCO2 for moist air conditions at the equilibrator temperature (fCO2 of seawater) or the air/sea interface (fCO2 of air), the mole fraction of CO2 in dry air x1 must be corrected to the mole fraction of CO2 in moist air x'1. If the
gas/water interface can be regarded as being saturated with water vapor at the water temperature, the following equation holds:
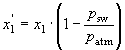 .
.
In this equation psw is the saturated water vapor pressure of seawater at the given temperature and patm is the total barometric pressure. Because of the thermal skin effect, the temperature at the interface is usually not the same as the mixed-layer bulk temperature (Schluessel et al. 1990). This effect, which typically accounts for an interface temperature of a few tenths of a degree below the bulk temperature, has significant implications for the effective fCO2 difference at the air/sea interface (Robertson and Watson 1992). As the exact skin temperature is rarely known, the bulk temperature is used instead. The fCO2 for moist air conditions can therefore be calculated according to
![]() .
.
The virial coefficient B (in cm3/mol) of CO2 can be calculated for the temperature range 265 to 320 K using a power series given by Weiss (1974):
![]() .
.
Weiss (1974) gives the following equation for the cross virial coefficient d of CO2 in air as a function of temperature (273 < T < 313 K):
![]() .
.
A.3 Correction of fCO2 to In Situ Temperature
To account for the slight warming of the seawater between the seawater intake and the equilibrator, the measured fCO2 values have to be corrected back to in situ temperature. Different equations (for pCO2 and fCO2) have been published in the literature (e.g., Gordon and Jones 1973; Weiss et al. 1982; Copin-Montegut 1988, 1989; Goyet et al. 1993; Takahashi et al. 1993). As the temperature changes are of the order of a few tenths of a degree only, the choice among these equations is not critical. We have used the following equation given by Weiss et al. (1982), which describes the temperature dependence of the solubility of CO2 and the carbonic acid equilibria:
![]() ,
,
where t is the seawater temperature (in °C). The equation gives the change in the logarithm of the fugacity of CO2 in moist air for an incremental increase of the temperature between in situ and measurement temperature.
